BiDil has no therapeutic bioequivalent
BiDil is the only fixed-dose combination approved by the US Food and Drug Administration (FDA) for use by African American patients in addition to routine HF medicines. The FDA has stated that there is no therapeutically bioequivalent substitute for BiDil. Additionally, neither approved labeling for isosorbide dinitrate nor hydralazine HCl products contain information about their use in the treatment of HF.1
- Pharmacokinetic data show that the fixed-dose combination of BiDil has different blood/plasma concentrations in the body than when the 2 medications are dosed separately
Review of pharmacokinetic data2
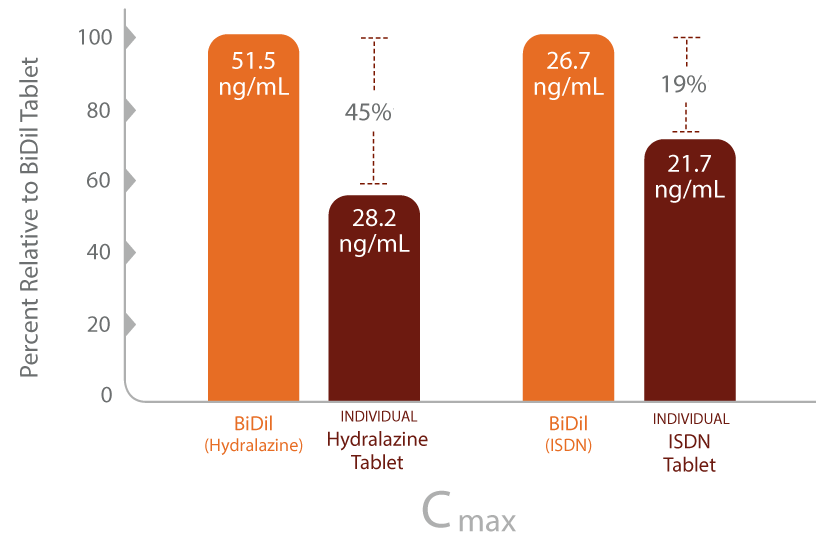
Cmax=maximum plasma concentration in the body; HYD=hydralazine; ISDN=isosorbide dinitrate.
Please click here to see full Prescribing Information for BiDil.
Individual components may not be the answer
Dosing isosorbide dinitrate and hydralazine separately is not an FDA recognized therapeutically bioequivalent approach to administering BiDil.1 Additionally, it may add unnecessary complexity and confusion to an already overwhelming burden for the HF patient.
From a physician standpoint, the 2 generic ingredients necessary to help patients achieve the results seen in the African American Heart Failure Trial (A-HeFT) simply are not available in the dosage strengths used in the fixed-dose combination.1,2
Daily dosing for separate components3,4

Request BiDil SavingsGet BiDil Savings for your patients.
INDICATIONS AND USAGE
BiDil is indicated for the treatment of heart failure as an adjunct to standard therapy in self-identified black patients to improve survival, to prolong time to hospitalization for heart failure, and to improve patient-reported functional status. There is little experience in patients with NYHA class IV heart failure. Most patients in the clinical trial supporting effectiveness (A-HeFT) received a loop diuretic, an angiotensin converting enzyme inhibitor or an angiotensin II receptor blocker, and a beta blocker, and many also received a cardiac glycoside or an aldosterone antagonist.
IMPORTANT SAFETY INFORMATION
BiDil is contraindicated in patients who are allergic to organic nitrates, or who take phosphodiesterase type 5 (PDE5) inhibitors, such as avanafil, sildenafil, tadalafil, or vardenafil, or soluble guanylate cyclase (sGC) stimulator (riociguat). Concomitant use can cause hypotension.
WARNINGS AND PRECAUTIONS
Hydralazine hydrochloride has been reported to cause a drug-induced systemic lupus erythematosus (SLE) syndrome. Symptoms and signs usually regress when hydralazine hydrochloride is discontinued.
Symptomatic hypotension, particularly with upright posture, may occur with even small doses of BiDil. Hypotension is most likely to occur in patients who have been volume or salt depleted; correct prior to initiation of BiDil. Hydralazine hydrochloride can cause tachycardia and hypotension potentially leading to myocardial ischemia and angina, particularly in patients with hypertrophic cardiomyopathy.
Hydralazine hydrochloride has been associated with peripheral neuritis, evidenced by paresthesia, numbness, and tingling, which may be related to an antipyridoxine effect. Pyridoxine should be added to BiDil therapy if such symptoms develop.
ADVERSE REACTIONS
Most common adverse reactions (> 5% more on BiDil than on placebo) were headache and dizziness.
The full Prescribing Information for BiDil is available here.
References: 1. Data on file. Arbor Pharmaceuticals, LLC. 2. Tam SW, Sabolinski ML, Worcel M, Packer M, Cohn JN. Lack of bioequivalence between different formulations of isosorbide dinitrate and hydralazine and the fixed-dose combination of isosorbide dinitrate/hydralazine: the V-HeFT paradox. Clin Pharmacokinet. 2007;46(10):885-895. 3. BiDil [package insert]. Atlanta, GA: Arbor Pharmaceuticals, Inc; 2015. 4. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines J Am Coll Cardiol. 2013;62(16):e147-e239.

